Themes & Goals
Human memory is well specified by cognitive principles such as encoding, mnemonic search, recall, proactive interference, and retrieval induced forgetting. In contrast, the processing dynamics behind these operations take place at the circuit level and remain woefully unspecified. Pathologies of memory associated with Alzheimer’s Disease, post-traumatic stress disorder, schizophrenia, autism and normal aging place an enormous burden on individuals, relationships and societies. The development of treatments and cures for these pathologies require a functional understanding of the circuit-based mechanisms of memory.
More generally, this research aims to develop a systems-level understanding of how memories are encoded and retrieved. One outcome will be the development of a ‘functional lens’ through which psychological disorders can be understood and diagnosed, analogous to the microscope used by Alois Alzheimer to identify plaques and tangles in his famous patient. Toward this end, the lab seeks to develop new tools to observe and track neural activation.
Approaches

High-density
electrophysiology
Circuit
manipulation
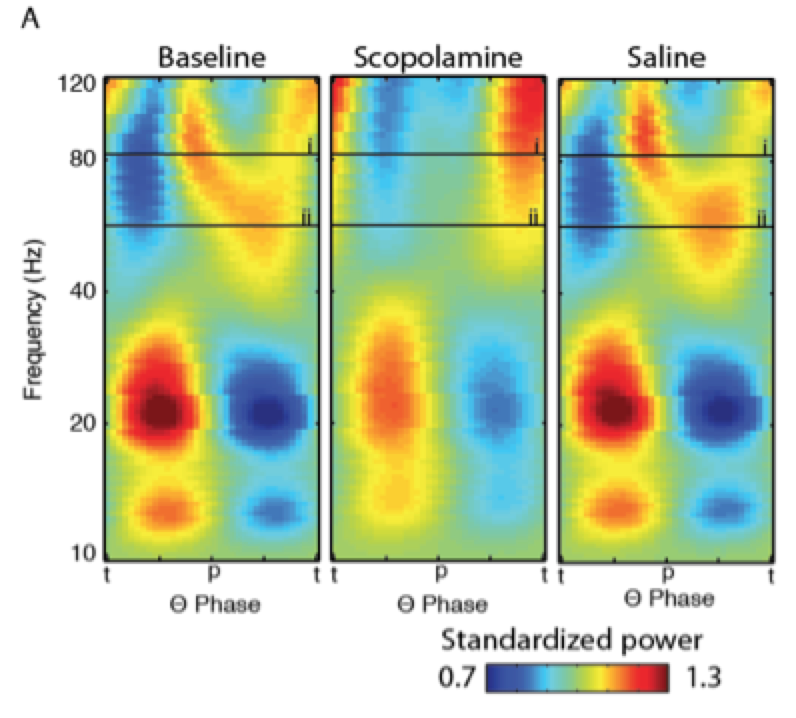
Analytical
techniques
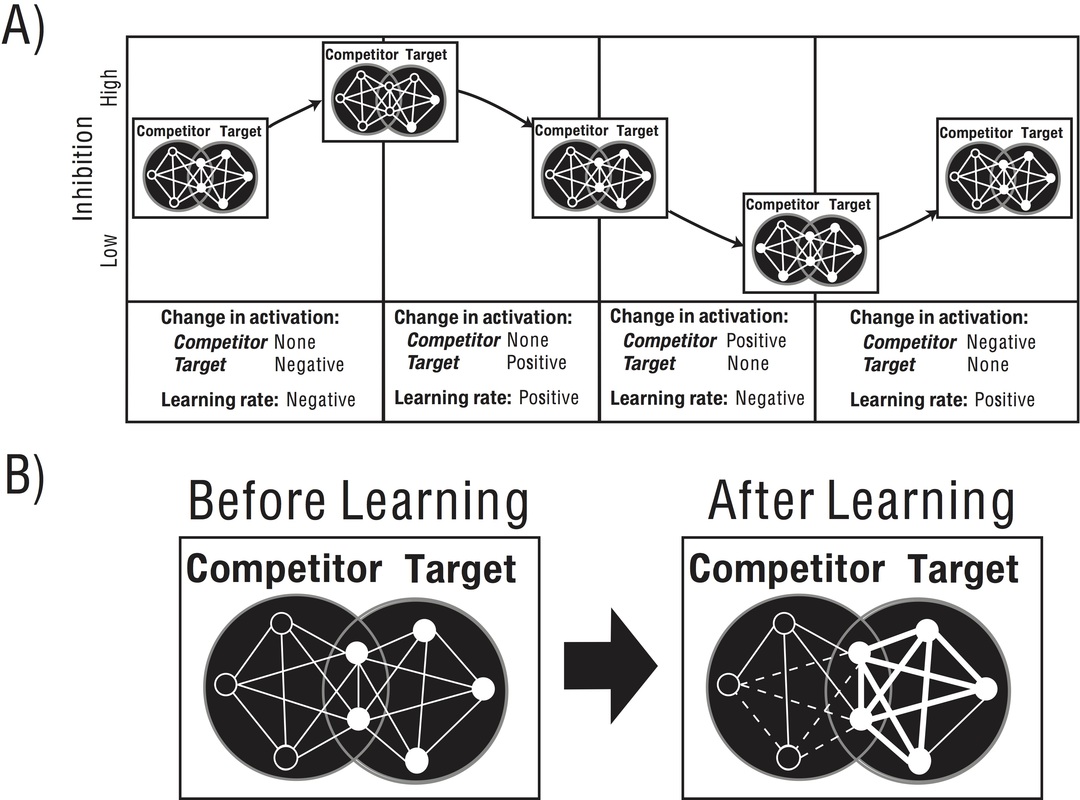
Computational
models
High-density electrophysiology generates a spatially and temporally precise record of neural activity. Arrays of tetrodes and depth-probes make it possible to track the spiking of tens to hundreds of isolated neurons and the aggregate synaptic input as reflected in the local field potential (LFP) simultaneously. In our research, we compare population activity to behavior and LFP to identify the principles of neural information processing that are central to memory. For example, I found that the tuning of entorhinal grid cells increases during epochs of increased hippocampal output, suggesting that this increase reflects the influence of mnemonic retrieval (Newman & Hasselmo, 2014; Newman et al., 2014).
Circuit manipulations help identify the physiological bases of neural information processing by reversibly suppressing or facilitating specific circuit componentTs. A standard method is to introduce a receptor activated by light (optogenetics) or a designer drug (DREADDs) into select cells that can be used to temporarily stimulate or silence action potentials. Pharmacology can also manipulate specific signaling pathways. Our research uses these methods to identify the role of acetylcholine in governing mnemonic processing. For example, I showed for the first time that spatial coding by entorhinal grid cells is dependent upon cholinergic modulation highlighting the importance of acetylcholine for coupling neural processing dynamics to behavior (Newman et al., 2014).
Analytical techniques bring rich meaning to electrophysiological data sets. Examples include spectral methods to extract the structure embedded in distributed time-varying signals and pattern classifiers to map the relationship between activation dynamics and behavior. Our research uses these techniques to translate electrophysiological recordings into measures of relevance for memory research. For example, we identified a form of phase-amplitude coupling in the entorhinal cortex using spectral analyses that may reflect mnemonic encoding (Newman et al., 2013). Another project mapped the relationship between memory strength and representation-level activation dynamics as identified via a pattern-classification analysis of human scalp EEG (Newman et al., 2010).
Computational models translate empirical observations into concrete principles, bridge disconnected literatures, and motivate new empirical studies. We use models to bridge neural physiology, as understood from in vitro studies, to memory, as understood from human behavior (Newman, Shay & Hasselmo, 2012). A recently developed model to account for intracellular recordings of grid cells that explains how networks of distinct neural populations cooperate to track animal behavior (Onslow, Hasselmo, & Newman, 2014). In another model, rhythmic fluctuations of inhibition have been shown to drive new learning and account for particularities of human memory (Norman*, Newman*, Detre & Polyn, 2006; Norman, Newman & Detre, 2007; Norman, Newman, & Perotte, 2005).